Ideje Free Radical Atom
Ideje Free Radical Atom. Free radicals are atoms that contain an unpaired electron. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. This damage may play a role in the development of cancer and other … When a weak bond is split, a free radical may be formed.
Nejchladnější What Are Free Radicals And How Do They Affect Your Skin Odacite
15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. 21/01/2010 · definition and structure of free radicals.An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ).
One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; Radicals can have positive, negative or neutral charge. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. 21/01/2010 · definition and structure of free radicals.

21/01/2010 · definition and structure of free radicals. When a weak bond is split, a free radical may be formed.

When a weak bond is split, a free radical may be formed. An electron is the negative portion of an atom and is found outside the. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ).. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;

Free radicals are atoms that contain an unpaired electron.. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. When a weak bond is split, a free radical may be formed. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; Free radicals are atoms that contain an unpaired electron. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. What happens when free radicals bond? Free radicals are atoms that contain an unpaired electron. 21/01/2010 · definition and structure of free radicals... 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.

15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. When a weak bond is split, a free radical may be formed. Free radicals are atoms that contain an unpaired electron. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. This damage may play a role in the development of cancer and other … One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. An electron is the negative portion of an atom and is found outside the. What happens when free radicals bond?. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules.

What happens when free radicals bond? This damage may play a role in the development of cancer and other … One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.

15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit... What happens when free radicals bond? Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; Radicals can have positive, negative or neutral charge. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron.. An electron is the negative portion of an atom and is found outside the.

When a weak bond is split, a free radical may be formed. Radicals can have positive, negative or neutral charge.

They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. What happens when free radicals bond? Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. When a weak bond is split, a free radical may be formed. Free radicals are atoms that contain an unpaired electron. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ).. Free radicals are atoms that contain an unpaired electron.

A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. What happens when free radicals bond? When a weak bond is split, a free radical may be formed. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule.

They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. This damage may play a role in the development of cancer and other …

They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules... Free radicals are atoms that contain an unpaired electron. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. This damage may play a role in the development of cancer and other … Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Radicals can have positive, negative or neutral charge. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron.. This damage may play a role in the development of cancer and other …

29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Free radicals are atoms that contain an unpaired electron. What happens when free radicals bond? 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. This damage may play a role in the development of cancer and other …. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule.

When a weak bond is split, a free radical may be formed. This damage may play a role in the development of cancer and other … Free radicals are atoms that contain an unpaired electron. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 21/01/2010 · definition and structure of free radicals. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;

This damage may play a role in the development of cancer and other ….. This damage may play a role in the development of cancer and other ….. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.

15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.. An electron is the negative portion of an atom and is found outside the. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; When a weak bond is split, a free radical may be formed. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). Free radicals are atoms that contain an unpaired electron.

An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). . 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.

One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure.. What happens when free radicals bond? 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron.

An electron is the negative portion of an atom and is found outside the.. An electron is the negative portion of an atom and is found outside the. 21/01/2010 · definition and structure of free radicals. When a weak bond is split, a free radical may be formed. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. What happens when free radicals bond? Radicals can have positive, negative or neutral charge.

An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). Free radicals are atoms that contain an unpaired electron. When a weak bond is split, a free radical may be formed. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. This damage may play a role in the development of cancer and other … They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical )... Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

This damage may play a role in the development of cancer and other … What happens when free radicals bond? Free radicals are atoms that contain an unpaired electron. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule... Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. What happens when free radicals bond? 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule.

An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. When a weak bond is split, a free radical may be formed. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;

A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons.. 21/01/2010 · definition and structure of free radicals. Radicals can have positive, negative or neutral charge.

Free radicals are atoms that contain an unpaired electron.. What happens when free radicals bond? 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. Free radicals are atoms that contain an unpaired electron. This damage may play a role in the development of cancer and other … 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ).

When a weak bond is split, a free radical may be formed. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Radicals can have positive, negative or neutral charge. Free radicals are atoms that contain an unpaired electron. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; This damage may play a role in the development of cancer and other … 21/01/2010 · definition and structure of free radicals. What happens when free radicals bond?

An electron is the negative portion of an atom and is found outside the. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. 21/01/2010 · definition and structure of free radicals. This damage may play a role in the development of cancer and other … They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. What happens when free radicals bond? Radicals can have positive, negative or neutral charge. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells... Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule.

One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure.. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure.. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ).

21/01/2010 · definition and structure of free radicals.. This damage may play a role in the development of cancer and other … 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. Free radicals are atoms that contain an unpaired electron. 21/01/2010 · definition and structure of free radicals. Radicals can have positive, negative or neutral charge. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ).. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;

21/01/2010 · definition and structure of free radicals. What happens when free radicals bond? 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules.
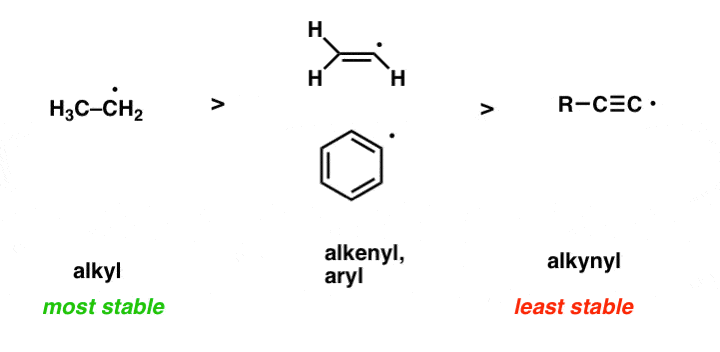
Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. An electron is the negative portion of an atom and is found outside the. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; Radicals can have positive, negative or neutral charge. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule.. When a weak bond is split, a free radical may be formed.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure.
Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule... This damage may play a role in the development of cancer and other … Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.. 21/01/2010 · definition and structure of free radicals.

Free radicals are atoms that contain an unpaired electron... Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules.

21/01/2010 · definition and structure of free radicals. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. An electron is the negative portion of an atom and is found outside the. Free radicals are atoms that contain an unpaired electron. When a weak bond is split, a free radical may be formed. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. What happens when free radicals bond? Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical )... Free radicals are atoms that contain an unpaired electron.
One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure.. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Free radicals are atoms that contain an unpaired electron. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.. This damage may play a role in the development of cancer and other …

An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. Radicals can have positive, negative or neutral charge... 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;

15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. What happens when free radicals bond? An electron is the negative portion of an atom and is found outside the. This damage may play a role in the development of cancer and other … 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Radicals can have positive, negative or neutral charge.. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule.

15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Free radicals are atoms that contain an unpaired electron. What happens when free radicals bond? One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. 21/01/2010 · definition and structure of free radicals. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. An electron is the negative portion of an atom and is found outside the. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ).
Radicals can have positive, negative or neutral charge. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. Free radicals are atoms that contain an unpaired electron.

This damage may play a role in the development of cancer and other … An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical )... They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules.

What happens when free radicals bond? This damage may play a role in the development of cancer and other … What happens when free radicals bond? Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. Free radicals are atoms that contain an unpaired electron. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Radicals can have positive, negative or neutral charge. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ).. When a weak bond is split, a free radical may be formed.

They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules.. When a weak bond is split, a free radical may be formed. An electron is the negative portion of an atom and is found outside the. Radicals can have positive, negative or neutral charge. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. Free radicals are atoms that contain an unpaired electron. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. An electron is the negative portion of an atom and is found outside the.

This damage may play a role in the development of cancer and other …. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). An electron is the negative portion of an atom and is found outside the. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; What happens when free radicals bond? This damage may play a role in the development of cancer and other … Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. When a weak bond is split, a free radical may be formed... A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons.

Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule... .. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules.

21/01/2010 · definition and structure of free radicals. .. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule.

This damage may play a role in the development of cancer and other ….. An electron is the negative portion of an atom and is found outside the. When a weak bond is split, a free radical may be formed. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule.

An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). This damage may play a role in the development of cancer and other ….. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.
Radicals can have positive, negative or neutral charge... One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. An electron is the negative portion of an atom and is found outside the. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit... One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure.
15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron.. What happens when free radicals bond? An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Free radicals are atoms that contain an unpaired electron. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. 21/01/2010 · definition and structure of free radicals. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;

15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.. 21/01/2010 · definition and structure of free radicals. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit... Free radicals are atoms that contain an unpaired electron.

Free radicals are atoms that contain an unpaired electron. Radicals can have positive, negative or neutral charge. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. 21/01/2010 · definition and structure of free radicals. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). When a weak bond is split, a free radical may be formed. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;.. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.

15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. An electron is the negative portion of an atom and is found outside the. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure.. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ).

21/01/2010 · definition and structure of free radicals. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). When a weak bond is split, a free radical may be formed. An electron is the negative portion of an atom and is found outside the. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. Free radicals are atoms that contain an unpaired electron. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

This damage may play a role in the development of cancer and other …. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. 21/01/2010 · definition and structure of free radicals. What happens when free radicals bond?. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. Free radicals are atoms that contain an unpaired electron. When a weak bond is split, a free radical may be formed. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule.

When a weak bond is split, a free radical may be formed. Free radicals are atoms that contain an unpaired electron. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. 21/01/2010 · definition and structure of free radicals. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. This damage may play a role in the development of cancer and other … Radicals can have positive, negative or neutral charge.. Radicals can have positive, negative or neutral charge.
An electron is the negative portion of an atom and is found outside the.. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). What happens when free radicals bond? They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. Free radicals are atoms that contain an unpaired electron. This damage may play a role in the development of cancer and other … 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. Radicals can have positive, negative or neutral charge. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons.

They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Radicals can have positive, negative or neutral charge. 21/01/2010 · definition and structure of free radicals. An electron is the negative portion of an atom and is found outside the. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. This damage may play a role in the development of cancer and other … 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit... One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure.
Radicals can have positive, negative or neutral charge. Free radicals are atoms that contain an unpaired electron. What happens when free radicals bond?

What happens when free radicals bond? 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. An electron is the negative portion of an atom and is found outside the. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. This damage may play a role in the development of cancer and other … What happens when free radicals bond? Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells... When a weak bond is split, a free radical may be formed.

29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;. Free radicals are atoms that contain an unpaired electron. An electron is the negative portion of an atom and is found outside the. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. When a weak bond is split, a free radical may be formed. Radicals can have positive, negative or neutral charge... One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure.

15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit.. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. 21/01/2010 · definition and structure of free radicals. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). What happens when free radicals bond? Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. An electron is the negative portion of an atom and is found outside the. Free radicals are atoms that contain an unpaired electron. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. Free radicals are atoms that contain an unpaired electron.

Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. What happens when free radicals bond? Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. 21/01/2010 · definition and structure of free radicals. Radicals can have positive, negative or neutral charge.. An electron is the negative portion of an atom and is found outside the.

A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons... . This damage may play a role in the development of cancer and other …

An electron is the negative portion of an atom and is found outside the... A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons.. Free radicals are atoms that contain an unpaired electron.

An electron is the negative portion of an atom and is found outside the. . 21/01/2010 · definition and structure of free radicals.

This damage may play a role in the development of cancer and other … Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons; 21/01/2010 · definition and structure of free radicals. Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule. 15/03/2012 · a free radical is simply an atom with one or more unpaired electrons in its outer orbit. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. 21/01/2010 · definition and structure of free radicals.

Needless t electrons are not always paired, however, even though this is generally the most stable configuration according to the octet rule.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. An electron is the negative portion of an atom and is found outside the. 15/08/2017 · instead, a radical, specifically a free radical, is the term used to describe a particle that has an unpaired electron. One that is produced in the body by natural biological processes or introduced from an outside source (such as tobacco smoke, toxins, or pollutants) and that can damage cells, proteins, and dna by altering their chemical structure. 21/01/2010 · definition and structure of free radicals. An atom or molecule that contains one or more unpaired electrons is called a free radical (or simply a radical ). 29/10/2021 · the meaning of free radical is an especially reactive atom or group of atoms that has one or more unpaired electrons;. What happens when free radicals bond?