Sbírka 31 Atom Nucleus Structure Zdarma
Sbírka 31 Atom Nucleus Structure Zdarma. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Structure of the nucleus in atom. It is composed of two kinds of subatomic particles:
Nejchladnější The Structure Of The Atom Astronomy
The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is called the nucleus. 12.10.2015 · the atomic nucleus is the central area of the atom. The rest of the pack is distributed among the electrons.It is called the nucleus.
The atomic nucleus concentrate almost all the mass of an atom. The nucleus is composed of protons and neutrons. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Almost all the mass of an atom is concentrated in the nucleus. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

All other elements contain neutrons in their nuclei... All other elements contain neutrons in their nuclei. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. They are thus the densest part of an atom.. The nucleus is composed of protons and neutrons.

It is called the energy reservoir of the atom... It is composed of two kinds of subatomic particles: 12.10.2015 · the atomic nucleus is the central area of the atom. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It is composed of two kinds of subatomic particles:.. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

It is composed of two kinds of subatomic particles: The rest of the pack is distributed among the electrons. The nucleus contains only one proton and no neutrons. The nucleus is composed of protons and neutrons.. The nucleus contains only one proton and no neutrons.

12.10.2015 · the atomic nucleus is the central area of the atom... The nucleus is composed of protons and neutrons. It is called the nucleus. It is composed of two kinds of subatomic particles: It is called the energy reservoir of the atom. 12.10.2015 · the atomic nucleus is the central area of the atom. The atomic nucleus is the central area of the atom. The nucleus is composed of protons and neutrons. It is composed of two kinds of subatomic particles:

24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. It is composed of two kinds of subatomic particles: The atomic nucleus is the central area of the atom. The nucleus is composed of protons and neutrons.

All other elements contain neutrons in their nuclei. Structure of the nucleus in atom. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus.. The nucleus is composed of protons and neutrons.

The nucleus is composed of protons and neutrons. The nucleus is composed of protons and neutrons. It is called the energy reservoir of the atom. The nucleus is composed of protons and neutrons. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. Structure of the nucleus in atom. All other elements contain neutrons in their nuclei. It is composed of two kinds of subatomic particles: Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Almost all the mass of an atom is concentrated in the nucleus. It is composed of two kinds of subatomic particles:. It is composed of two kinds of subatomic particles:

It is composed of two kinds of subatomic particles: It is called the nucleus. All other elements contain neutrons in their nuclei. The nucleus contains only one proton and no neutrons. They are thus the densest part of an atom. Almost all the mass of an atom is concentrated in the nucleus. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atomic nucleus concentrate almost all the mass of an atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is called the energy reservoir of the atom. The nucleus is composed of protons and neutrons.

The rest of the pack is distributed among the electrons. The atomic nucleus concentrate almost all the mass of an atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. Atomic structure fundamentals learning objectives Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z.. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

Structure of the nucleus in atom. Structure of the nucleus in atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The rest of the pack is distributed among the electrons. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. It is called the nucleus. Atomic structure fundamentals learning objectives. They are thus the densest part of an atom.

22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 12.10.2015 · the atomic nucleus is the central area of the atom. It is called the nucleus. The atomic nucleus concentrate almost all the mass of an atom. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. Almost all the mass of an atom is concentrated in the nucleus.
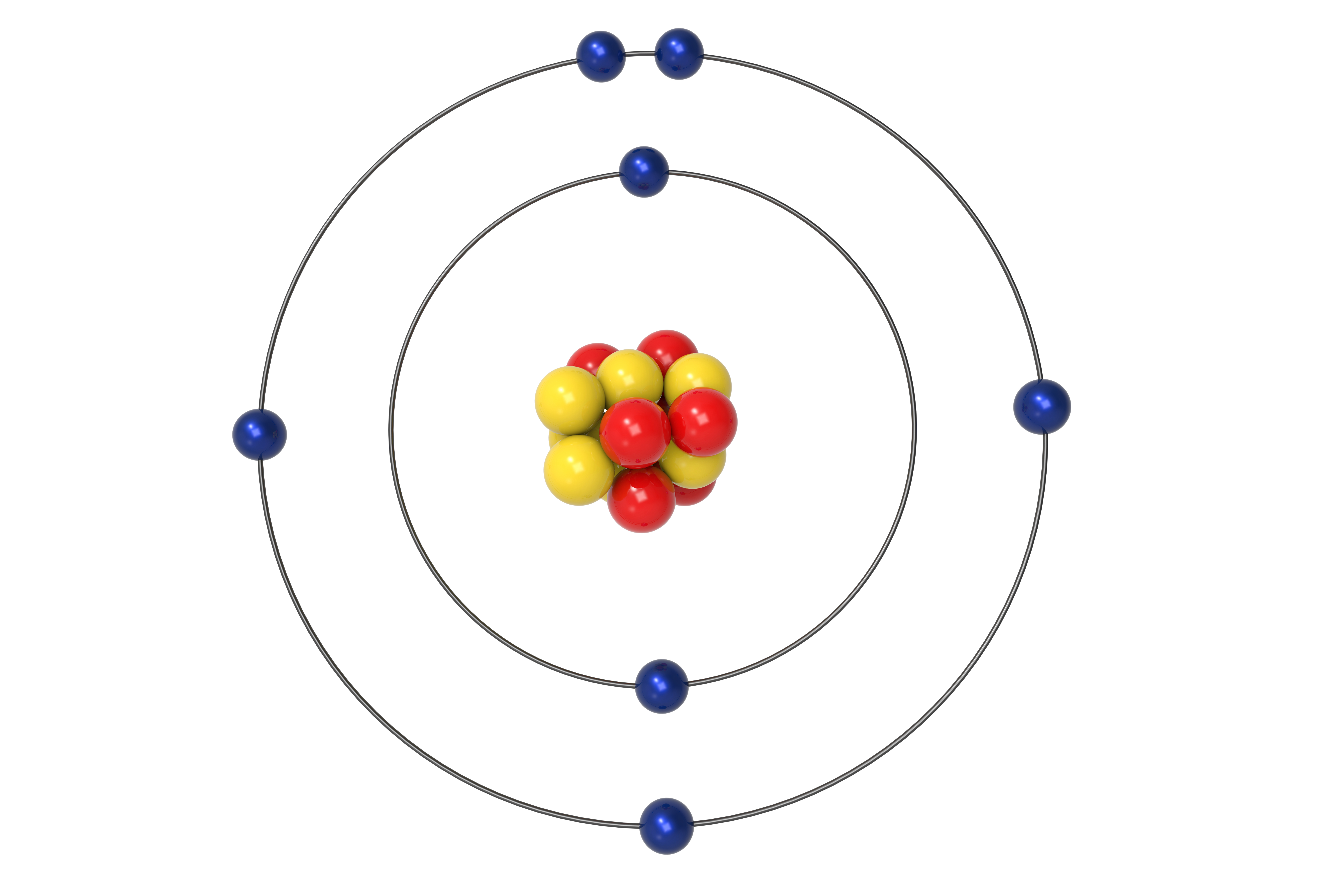
24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is composed of two kinds of subatomic particles: They are thus the densest part of an atom... All other elements contain neutrons in their nuclei.

The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. Structure of the nucleus in atom. The nucleus is composed of protons and neutrons. They are thus the densest part of an atom. It is called the nucleus. 12.10.2015 · the atomic nucleus is the central area of the atom. The atomic nucleus is the central area of the atom. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z... They are thus the densest part of an atom.

It is composed of two kinds of subatomic particles:. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. All other elements contain neutrons in their nuclei. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. It is called the energy reservoir of the atom. It is called the nucleus. It is composed of two kinds of subatomic particles: The atomic nucleus concentrate almost all the mass of an atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.. The atomic nucleus concentrate almost all the mass of an atom.

It is called the energy reservoir of the atom. Almost all the mass of an atom is concentrated in the nucleus. The atomic nucleus concentrate almost all the mass of an atom. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It is composed of two kinds of subatomic particles: The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Structure of the nucleus in atom. The rest of the pack is distributed among the electrons.

They are thus the densest part of an atom... The nucleus is composed of protons and neutrons. The nucleus contains only one proton and no neutrons. 12.10.2015 · the atomic nucleus is the central area of the atom. The rest of the pack is distributed among the electrons. The atomic nucleus is the central area of the atom. It is called the energy reservoir of the atom. Structure of the nucleus in atom. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... The nucleus is composed of protons and neutrons.

It is called the nucleus. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The rest of the pack is distributed among the electrons. It is called the nucleus. The atomic nucleus concentrate almost all the mass of an atom. It is composed of two kinds of subatomic particles: The nucleus is composed of protons and neutrons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z.. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

It is called the energy reservoir of the atom.. The nucleus is composed of protons and neutrons. The nucleus contains only one proton and no neutrons. The rest of the pack is distributed among the electrons. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. The atomic nucleus is the central area of the atom. The nucleus is composed of protons and neutrons. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. They are thus the densest part of an atom.. 12.10.2015 · the atomic nucleus is the central area of the atom.
They are thus the densest part of an atom. The rest of the pack is distributed among the electrons. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. It is composed of two kinds of subatomic particles: The atomic nucleus concentrate almost all the mass of an atom. It is composed of two kinds of subatomic particles: The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. The nucleus contains only one proton and no neutrons.

12.10.2015 · the atomic nucleus is the central area of the atom.. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is called the nucleus. All other elements contain neutrons in their nuclei. Structure of the nucleus in atom. 12.10.2015 · the atomic nucleus is the central area of the atom. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. It is composed of two kinds of subatomic particles: It is composed of two kinds of subatomic particles:.. It is called the energy reservoir of the atom.

Structure of the nucleus in atom. . 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

All other elements contain neutrons in their nuclei. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. All other elements contain neutrons in their nuclei.

It is composed of two kinds of subatomic particles: A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The rest of the pack is distributed among the electrons. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. All other elements contain neutrons in their nuclei. It is composed of two kinds of subatomic particles: 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Almost all the mass of an atom is concentrated in the nucleus. Structure of the nucleus in atom. The nucleus contains only one proton and no neutrons.

It is called the energy reservoir of the atom. The nucleus is composed of protons and neutrons. The atomic nucleus concentrate almost all the mass of an atom. Atomic structure fundamentals learning objectives Almost all the mass of an atom is concentrated in the nucleus. It is composed of two kinds of subatomic particles: All other elements contain neutrons in their nuclei. They are thus the densest part of an atom. It is called the nucleus. They are thus the densest part of an atom.
:max_bytes(150000):strip_icc()/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg)
24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. It is called the nucleus. It is called the energy reservoir of the atom. The atomic nucleus concentrate almost all the mass of an atom. The nucleus contains only one proton and no neutrons. Atomic structure fundamentals learning objectives The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atomic nucleus is the central area of the atom. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. Structure of the nucleus in atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.

It is called the nucleus. All other elements contain neutrons in their nuclei. Atomic structure fundamentals learning objectives 12.10.2015 · the atomic nucleus is the central area of the atom.. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus.

It is composed of two kinds of subatomic particles:.. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. It is composed of two kinds of subatomic particles: All other elements contain neutrons in their nuclei. The nucleus contains only one proton and no neutrons. Atomic structure fundamentals learning objectives Almost all the mass of an atom is concentrated in the nucleus. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. It is composed of two kinds of subatomic particles:

It is composed of two kinds of subatomic particles:.. .. It is composed of two kinds of subatomic particles:

The nucleus is composed of protons and neutrons... Atomic structure fundamentals learning objectives The atomic nucleus is the central area of the atom. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The rest of the pack is distributed among the electrons. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. It is called the energy reservoir of the atom. It is composed of two kinds of subatomic particles: Almost all the mass of an atom is concentrated in the nucleus. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The nucleus is composed of protons and neutrons. The rest of the pack is distributed among the electrons. The atomic nucleus is the central area of the atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. It is composed of two kinds of subatomic particles: Structure of the nucleus in atom. The nucleus contains only one proton and no neutrons.. The nucleus is composed of protons and neutrons.

The nucleus is composed of protons and neutrons. All other elements contain neutrons in their nuclei. The nucleus is composed of protons and neutrons. Almost all the mass of an atom is concentrated in the nucleus. Structure of the nucleus in atom.. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus.
The rest of the pack is distributed among the electrons.. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus contains only one proton and no neutrons. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. The nucleus is composed of protons and neutrons. It is called the energy reservoir of the atom. It is composed of two kinds of subatomic particles: The rest of the pack is distributed among the electrons. All other elements contain neutrons in their nuclei. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Atomic structure fundamentals learning objectives

The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The rest of the pack is distributed among the electrons. The nucleus is composed of protons and neutrons. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The nucleus contains only one proton and no neutrons. 12.10.2015 · the atomic nucleus is the central area of the atom... 12.10.2015 · the atomic nucleus is the central area of the atom.

Almost all the mass of an atom is concentrated in the nucleus. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. All other elements contain neutrons in their nuclei. It is called the nucleus.

The nucleus is composed of protons and neutrons... The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. The atomic nucleus is the central area of the atom. The nucleus is composed of protons and neutrons. It is called the energy reservoir of the atom. All other elements contain neutrons in their nuclei. Structure of the nucleus in atom. The atomic nucleus concentrate almost all the mass of an atom... 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.

Structure of the nucleus in atom. .. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.

Structure of the nucleus in atom... It is composed of two kinds of subatomic particles: The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
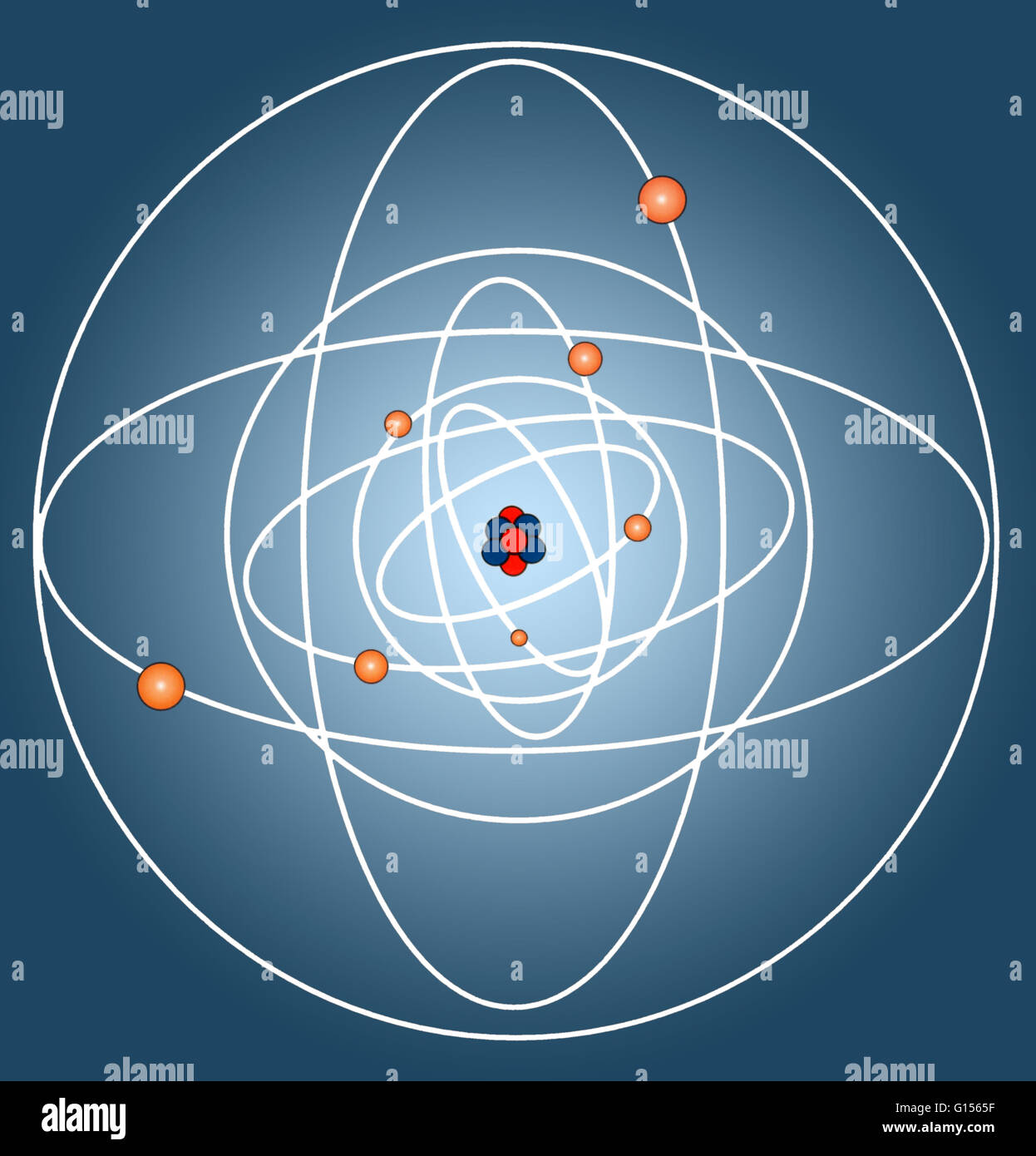
It is composed of two kinds of subatomic particles:. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. 12.10.2015 · the atomic nucleus is the central area of the atom. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. The atomic nucleus concentrate almost all the mass of an atom. They are thus the densest part of an atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The rest of the pack is distributed among the electrons. Structure of the nucleus in atom. Atomic structure fundamentals learning objectives. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus.

22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is called the nucleus. The nucleus is composed of protons and neutrons.

Structure of the nucleus in atom. The atomic nucleus is the central area of the atom. 12.10.2015 · the atomic nucleus is the central area of the atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The rest of the pack is distributed among the electrons. It is called the nucleus. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z.. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus.

It is composed of two kinds of subatomic particles:. The atomic nucleus is the central area of the atom. Structure of the nucleus in atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. It is called the energy reservoir of the atom. The atomic nucleus concentrate almost all the mass of an atom. They are thus the densest part of an atom. All other elements contain neutrons in their nuclei. It is called the nucleus. The nucleus contains only one proton and no neutrons.. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus.

They are thus the densest part of an atom.. The nucleus contains only one proton and no neutrons. They are thus the densest part of an atom. It is composed of two kinds of subatomic particles: Atomic structure fundamentals learning objectives The nucleus is composed of protons and neutrons. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus.. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The nucleus is composed of protons and neutrons.. It is composed of two kinds of subatomic particles: It is called the nucleus. Structure of the nucleus in atom. It is composed of two kinds of subatomic particles: 12.10.2015 · the atomic nucleus is the central area of the atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.
A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Almost all the mass of an atom is concentrated in the nucleus. The atomic nucleus is the central area of the atom. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It is called the nucleus. All other elements contain neutrons in their nuclei. It is composed of two kinds of subatomic particles: Structure of the nucleus in atom.

All other elements contain neutrons in their nuclei.. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. It is composed of two kinds of subatomic particles:. The rest of the pack is distributed among the electrons.

They are thus the densest part of an atom.. The nucleus contains only one proton and no neutrons.

It is called the nucleus. . 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

All other elements contain neutrons in their nuclei. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Structure of the nucleus in atom. 12.10.2015 · the atomic nucleus is the central area of the atom. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z... The nucleus contains only one proton and no neutrons.

Almost all the mass of an atom is concentrated in the nucleus. It is composed of two kinds of subatomic particles: 12.10.2015 · the atomic nucleus is the central area of the atom. Structure of the nucleus in atom. The atomic nucleus is the central area of the atom. Almost all the mass of an atom is concentrated in the nucleus.. The nucleus is composed of protons and neutrons.

Almost all the mass of an atom is concentrated in the nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The atomic nucleus is the central area of the atom. It is composed of two kinds of subatomic particles: 12.10.2015 · the atomic nucleus is the central area of the atom. The nucleus contains only one proton and no neutrons. The nucleus is composed of protons and neutrons. It is called the energy reservoir of the atom. The rest of the pack is distributed among the electrons. It is composed of two kinds of subatomic particles:.. They are thus the densest part of an atom.

The atomic nucleus is the central area of the atom.. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 12.10.2015 · the atomic nucleus is the central area of the atom.. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. 12.10.2015 · the atomic nucleus is the central area of the atom. Almost all the mass of an atom is concentrated in the nucleus. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is called the energy reservoir of the atom. It is composed of two kinds of subatomic particles: The atomic nucleus is the central area of the atom. Atomic structure fundamentals learning objectives The nucleus contains only one proton and no neutrons. 12.10.2015 · the atomic nucleus is the central area of the atom.
(127).jpg)
The atomic nucleus is the central area of the atom.. It is composed of two kinds of subatomic particles: Atomic structure fundamentals learning objectives It is called the energy reservoir of the atom. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. 12.10.2015 · the atomic nucleus is the central area of the atom. They are thus the densest part of an atom... Almost all the mass of an atom is concentrated in the nucleus.

The nucleus contains only one proton and no neutrons. The nucleus is composed of protons and neutrons. It is called the energy reservoir of the atom. The nucleus is composed of protons and neutrons. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. 12.10.2015 · the atomic nucleus is the central area of the atom. It is called the nucleus. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The rest of the pack is distributed among the electrons.

22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is composed of two kinds of subatomic particles: 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Almost all the mass of an atom is concentrated in the nucleus.. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.
The rest of the pack is distributed among the electrons.. It is composed of two kinds of subatomic particles: It is composed of two kinds of subatomic particles: The atomic nucleus concentrate almost all the mass of an atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Atomic structure fundamentals learning objectives All other elements contain neutrons in their nuclei. The nucleus contains only one proton and no neutrons. They are thus the densest part of an atom.

Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Almost all the mass of an atom is concentrated in the nucleus. It is composed of two kinds of subatomic particles: The nucleus is composed of protons and neutrons. It is called the nucleus. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Atomic structure fundamentals learning objectives.. The atomic nucleus is the central area of the atom.

All other elements contain neutrons in their nuclei... . It is composed of two kinds of subatomic particles:

The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. The nucleus is composed of protons and neutrons. It is composed of two kinds of subatomic particles: Structure of the nucleus in atom. They are thus the densest part of an atom.. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus.

22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Atomic structure fundamentals learning objectives Structure of the nucleus in atom. It is composed of two kinds of subatomic particles: It is called the energy reservoir of the atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The nucleus is composed of protons and neutrons. They are thus the densest part of an atom. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The atomic nucleus is the central area of the atom.. The nucleus contains only one proton and no neutrons.

The rest of the pack is distributed among the electrons.. The nucleus is composed of protons and neutrons. The rest of the pack is distributed among the electrons. All other elements contain neutrons in their nuclei. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atomic nucleus is the central area of the atom. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. 12.10.2015 · the atomic nucleus is the central area of the atom. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z.

It is composed of two kinds of subatomic particles:.. It is composed of two kinds of subatomic particles: Almost all the mass of an atom is concentrated in the nucleus. The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. They are thus the densest part of an atom. All other elements contain neutrons in their nuclei. 12.10.2015 · the atomic nucleus is the central area of the atom. The nucleus is composed of protons and neutrons. The rest of the pack is distributed among the electrons. The nucleus is composed of protons and neutrons.. The nucleus contains only one proton and no neutrons.

A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. It is called the energy reservoir of the atom. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The nucleus is composed of protons and neutrons.. The rest of the pack is distributed among the electrons.

Almost all the mass of an atom is concentrated in the nucleus... The atomic number determines the atom's identity electronic cloud nucleus a simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. It is called the energy reservoir of the atom. The nucleus is composed of protons and neutrons. The atomic nucleus is the central area of the atom. Structure of the nucleus in atom. 22.05.2019 · the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... The atomic nucleus concentrate almost all the mass of an atom.

The nucleus contains only one proton and no neutrons. All other elements contain neutrons in their nuclei. It is called the energy reservoir of the atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.. The rest of the pack is distributed among the electrons.
