Atom Mass Number
Atom Mass Number. The mass number is determined by the number of protons and neutrons combined. Mass number is often denoted using a capital letter a. The mass number of an atom is its total number of protons and neutrons.
Nejlepší Relative Atomic Mass A Question Science By Degrees
20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons. Mass number is often denoted using a capital letter a.Mass number is often denoted using a capital letter a.
Mass number is often denoted using a capital letter a. The sum of the mass number and the atomic number for an atom … The mass number of an atom is its total number of protons and neutrons. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

The mass number reports the mass of the atom's nucleus in atomic mass units (amu)... 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is often denoted using a capital letter a. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The sum of the mass number and the atomic number for an atom … 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

The mass number reports the mass of the atom's nucleus in atomic mass units (amu)... In other words, it is the sum of the number of nucleons in an atom. The mass number of an atom is its total number of protons and neutrons. The sum of the mass number and the atomic number for an atom … Mass number is often denoted using a capital letter a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers , but they can be the same. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number is determined by the number of protons and neutrons combined.. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).

The sum of the mass number and the atomic number for an atom … The mass number of an atom is its total number of protons and neutrons. Mass number is often denoted using a capital letter a. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Atoms of different elements usually have different mass numbers , but they can be the same. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The sum of the mass number and the atomic number for an atom … The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.. The mass number of an atom is its total number of protons and neutrons.

14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. The sum of the mass number and the atomic number for an atom …

The sum of the mass number and the atomic number for an atom … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number of an atom is its total number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The mass number is determined by the number of protons and neutrons combined. Atoms of different elements usually have different mass numbers , but they can be the same.
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)
In other words, it is the sum of the number of nucleons in an atom.. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The sum of the mass number and the atomic number for an atom … The mass number is determined by the number of protons and neutrons combined... Atoms of different elements usually have different mass numbers , but they can be the same.

The mass number of an atom is its total number of protons and neutrons. . 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The mass number is determined by the number of protons and neutrons combined. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number is determined by the number of protons and neutrons combined. Mass number is often denoted using a capital letter a. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. The sum of the mass number and the atomic number for an atom ….. In other words, it is the sum of the number of nucleons in an atom.

The mass number is determined by the number of protons and neutrons combined. The mass number of an atom is its total number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a. The mass number is determined by the number of protons and neutrons combined. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The sum of the mass number and the atomic number for an atom … The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Atoms of different elements usually have different mass numbers , but they can be the same. In other words, it is the sum of the number of nucleons in an atom.
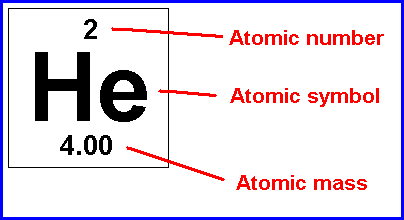
The mass number is determined by the number of protons and neutrons combined. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The sum of the mass number and the atomic number for an atom … In other words, it is the sum of the number of nucleons in an atom. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number is determined by the number of protons and neutrons combined. Mass number is often denoted using a capital letter a.

14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus... .. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number is determined by the number of protons and neutrons combined. In other words, it is the sum of the number of nucleons in an atom. The mass number of an atom is its total number of protons and neutrons. The sum of the mass number and the atomic number for an atom … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons. Mass number is often denoted using a capital letter a. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.
20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Mass number is often denoted using a capital letter a. The mass number of an atom is its total number of protons and neutrons. The sum of the mass number and the atomic number for an atom … 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. In other words, it is the sum of the number of nucleons in an atom.

The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number is determined by the number of protons and neutrons combined. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). In other words, it is the sum of the number of nucleons in an atom... 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons)... 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The mass number is determined by the number of protons and neutrons combined. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. In other words, it is the sum of the number of nucleons in an atom. The sum of the mass number and the atomic number for an atom … Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

The sum of the mass number and the atomic number for an atom … In other words, it is the sum of the number of nucleons in an atom. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The sum of the mass number and the atomic number for an atom … The mass number of an atom is its total number of protons and neutrons. The mass number is determined by the number of protons and neutrons combined. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number... In other words, it is the sum of the number of nucleons in an atom.

The sum of the mass number and the atomic number for an atom … In other words, it is the sum of the number of nucleons in an atom. The mass number of an atom is its total number of protons and neutrons.
20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons)... 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number is determined by the number of protons and neutrons combined.. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers , but they can be the same. The mass number is determined by the number of protons and neutrons combined. The mass number of an atom is its total number of protons and neutrons. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

The sum of the mass number and the atomic number for an atom ….. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a.

The mass number of an atom is its total number of protons and neutrons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The mass number is determined by the number of protons and neutrons combined. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number is determined by the number of protons and neutrons combined. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

The mass number of an atom is its total number of protons and neutrons.. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number is determined by the number of protons and neutrons combined. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). In other words, it is the sum of the number of nucleons in an atom. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. The mass number of an atom is its total number of protons and neutrons.

The mass number reports the mass of the atom's nucleus in atomic mass units (amu). .. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number is determined by the number of protons and neutrons combined. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number is determined by the number of protons and neutrons combined.

Mass number is often denoted using a capital letter a... In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The sum of the mass number and the atomic number for an atom … 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons)... 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The mass number of an atom is its total number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. The mass number is determined by the number of protons and neutrons combined. Mass number is often denoted using a capital letter a.. The sum of the mass number and the atomic number for an atom …

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is often denoted using a capital letter a. The sum of the mass number and the atomic number for an atom … The mass number is determined by the number of protons and neutrons combined. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number... Mass number is often denoted using a capital letter a.

The mass number is determined by the number of protons and neutrons combined. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number is determined by the number of protons and neutrons combined. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons.

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. In other words, it is the sum of the number of nucleons in an atom. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The mass number is determined by the number of protons and neutrons combined. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Mass number is often denoted using a capital letter a. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons. The sum of the mass number and the atomic number for an atom … Atoms of different elements usually have different mass numbers , but they can be the same.

Atoms of different elements usually have different mass numbers , but they can be the same.. Atoms of different elements usually have different mass numbers , but they can be the same. The sum of the mass number and the atomic number for an atom … The mass number is determined by the number of protons and neutrons combined. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number reports the mass of the atom's nucleus in atomic mass units (amu). In other words, it is the sum of the number of nucleons in an atom. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Mass number is often denoted using a capital letter a. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. The mass number of an atom is its total number of protons and neutrons.

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number of an atom is its total number of protons and neutrons. Mass number is often denoted using a capital letter a. The mass number is determined by the number of protons and neutrons combined. Atoms of different elements usually have different mass numbers , but they can be the same. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The sum of the mass number and the atomic number for an atom …. Mass number is often denoted using a capital letter a.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number is determined by the number of protons and neutrons combined. The mass number of an atom is its total number of protons and neutrons. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number... 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

In other words, it is the sum of the number of nucleons in an atom... The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number... 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a... 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

In other words, it is the sum of the number of nucleons in an atom... 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number of an atom is its total number of protons and neutrons. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The mass number is determined by the number of protons and neutrons combined. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same.

In other words, it is the sum of the number of nucleons in an atom.. The sum of the mass number and the atomic number for an atom … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same.. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).

Atoms of different elements usually have different mass numbers , but they can be the same.. Atoms of different elements usually have different mass numbers , but they can be the same. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The sum of the mass number and the atomic number for an atom … Mass number is often denoted using a capital letter a. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number of an atom is its total number of protons and neutrons. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number is determined by the number of protons and neutrons combined. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.
The mass number is determined by the number of protons and neutrons combined. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus... In other words, it is the sum of the number of nucleons in an atom.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The sum of the mass number and the atomic number for an atom … The mass number of an atom is its total number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers , but they can be the same. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number is determined by the number of protons and neutrons combined. Mass number is often denoted using a capital letter a.. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The mass number is determined by the number of protons and neutrons combined. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Mass number is often denoted using a capital letter a. The mass number of an atom is its total number of protons and neutrons.. The mass number of an atom is its total number of protons and neutrons.
In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number is determined by the number of protons and neutrons combined. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Mass number is often denoted using a capital letter a. The sum of the mass number and the atomic number for an atom … The mass number of an atom is its total number of protons and neutrons.. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The sum of the mass number and the atomic number for an atom … Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Mass number is often denoted using a capital letter a. The mass number of an atom is its total number of protons and neutrons. The mass number is determined by the number of protons and neutrons combined. Atoms of different elements usually have different mass numbers , but they can be the same... The mass number of an atom is its total number of protons and neutrons.
The mass number of an atom is its total number of protons and neutrons. . In other words, it is the sum of the number of nucleons in an atom.

Atoms of different elements usually have different mass numbers , but they can be the same. In other words, it is the sum of the number of nucleons in an atom. The sum of the mass number and the atomic number for an atom … Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons... Mass number is often denoted using a capital letter a.

The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. The sum of the mass number and the atomic number for an atom … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The mass number is determined by the number of protons and neutrons combined. Mass number is often denoted using a capital letter a.

The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The sum of the mass number and the atomic number for an atom ….. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.
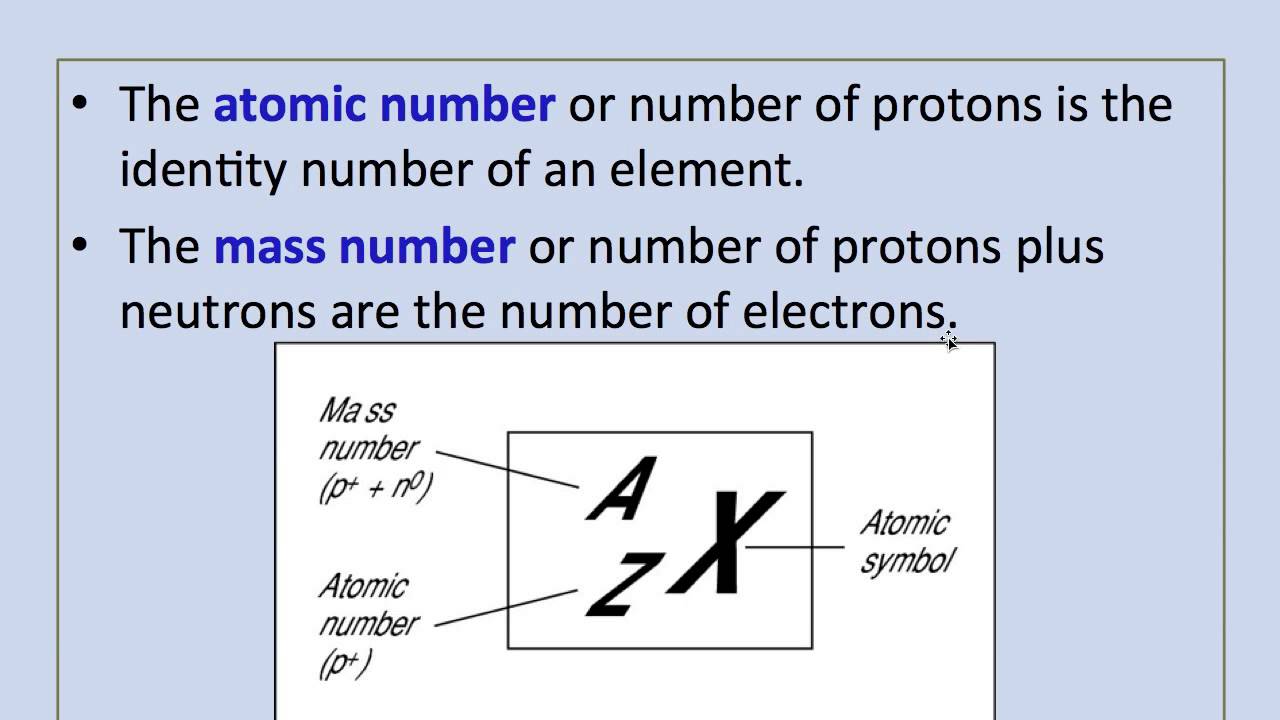
In other words, it is the sum of the number of nucleons in an atom. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same... 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

Mass number is often denoted using a capital letter a. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The mass number of an atom is its total number of protons and neutrons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number is determined by the number of protons and neutrons combined. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).

In other words, it is the sum of the number of nucleons in an atom... The mass number of an atom is its total number of protons and neutrons. The mass number is determined by the number of protons and neutrons combined. The sum of the mass number and the atomic number for an atom … In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The mass number of an atom is its total number of protons and neutrons. The sum of the mass number and the atomic number for an atom … Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

In other words, it is the sum of the number of nucleons in an atom. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Atoms of different elements usually have different mass numbers , but they can be the same. The mass number is determined by the number of protons and neutrons combined. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a. The sum of the mass number and the atomic number for an atom … Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. In other words, it is the sum of the number of nucleons in an atom.. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

Mass number is often denoted using a capital letter a. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

The sum of the mass number and the atomic number for an atom ….. Atoms of different elements usually have different mass numbers , but they can be the same. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a.

The sum of the mass number and the atomic number for an atom ….. Atoms of different elements usually have different mass numbers , but they can be the same. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number is determined by the number of protons and neutrons combined. Atoms of different elements usually have different mass numbers , but they can be the same.

20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.. The mass number is determined by the number of protons and neutrons combined. The mass number of an atom is its total number of protons and neutrons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. In other words, it is the sum of the number of nucleons in an atom. In other words, it is the sum of the number of nucleons in an atom.

Mass number is often denoted using a capital letter a... Atoms of different elements usually have different mass numbers , but they can be the same.. Mass number is often denoted using a capital letter a.

In other words, it is the sum of the number of nucleons in an atom... In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers , but they can be the same. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number of an atom is its total number of protons and neutrons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The mass number is determined by the number of protons and neutrons combined. The sum of the mass number and the atomic number for an atom …. In other words, it is the sum of the number of nucleons in an atom.

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The mass number is determined by the number of protons and neutrons combined. The mass number of an atom is its total number of protons and neutrons... Atoms of different elements usually have different mass numbers , but they can be the same.

In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The sum of the mass number and the atomic number for an atom … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The mass number is determined by the number of protons and neutrons combined. In other words, it is the sum of the number of nucleons in an atom. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Atoms of different elements usually have different mass numbers , but they can be the same.

Mass number is often denoted using a capital letter a... .. The mass number of an atom is its total number of protons and neutrons.

The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The sum of the mass number and the atomic number for an atom … 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Mass number is often denoted using a capital letter a.. Mass number is often denoted using a capital letter a.

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. .. In other words, it is the sum of the number of nucleons in an atom.

In other words, it is the sum of the number of nucleons in an atom.. In other words, it is the sum of the number of nucleons in an atom. The sum of the mass number and the atomic number for an atom … Mass number is often denoted using a capital letter a. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number is determined by the number of protons and neutrons combined. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

The sum of the mass number and the atomic number for an atom ….. . In other words, it is the sum of the number of nucleons in an atom.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number of an atom is its total number of protons and neutrons. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The sum of the mass number and the atomic number for an atom … Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number is determined by the number of protons and neutrons combined. Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons)... In other words, it is the sum of the number of nucleons in an atom.

In other words, it is the sum of the number of nucleons in an atom. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. The mass number of an atom is its total number of protons and neutrons. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.. Mass number is often denoted using a capital letter a. The mass number of an atom is its total number of protons and neutrons. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers , but they can be the same. The sum of the mass number and the atomic number for an atom … Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number is determined by the number of protons and neutrons combined. The sum of the mass number and the atomic number for an atom … The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Mass number is often denoted using a capital letter a. The mass number of an atom is its total number of protons and neutrons.. The mass number of an atom is its total number of protons and neutrons.
Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is often denoted using a capital letter a. The mass number is determined by the number of protons and neutrons combined. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. In other words, it is the sum of the number of nucleons in an atom.
20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Mass number is often denoted using a capital letter a. Atoms of different elements usually have different mass numbers , but they can be the same.

Atoms of different elements usually have different mass numbers , but they can be the same. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number is determined by the number of protons and neutrons combined. Mass number is often denoted using a capital letter a.. The mass number is determined by the number of protons and neutrons combined.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).. The sum of the mass number and the atomic number for an atom …
In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers , but they can be the same. The sum of the mass number and the atomic number for an atom … 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number is determined by the number of protons and neutrons combined.
The mass number is determined by the number of protons and neutrons combined. The mass number is determined by the number of protons and neutrons combined. The mass number of an atom is its total number of protons and neutrons... The mass number of an atom is its total number of protons and neutrons.

The mass number of an atom is its total number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons. Mass number is often denoted using a capital letter a. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. Atoms of different elements usually have different mass numbers , but they can be the same.